Fungicidal Power You Can Trust®
High-performance antifungal3,7
NAFTIN GEL 2% delivers the proven efficacy of an allylamine with the safety profile of vehicle3
- A fungicidal allylamine that attacks offending microorganisms—highest efficacy observed at 6 weeks (4 weeks post last treatment)3
- Adverse events comparable to vehicle3:
- Application site reactions were the most common adverse reactions seen in clinical trials with NAFTIN GEL 2% (2%) vs vehicle (1%)
Harnessing the power of a fungicidal allylamine in a 2% gel3
Clinically proven to clear interdigital tinea pedis even after treatment ends3
Primary endpoint
Complete cure at week 6 (4 weeks post last treatment) with NAFTIN GEL 2%3
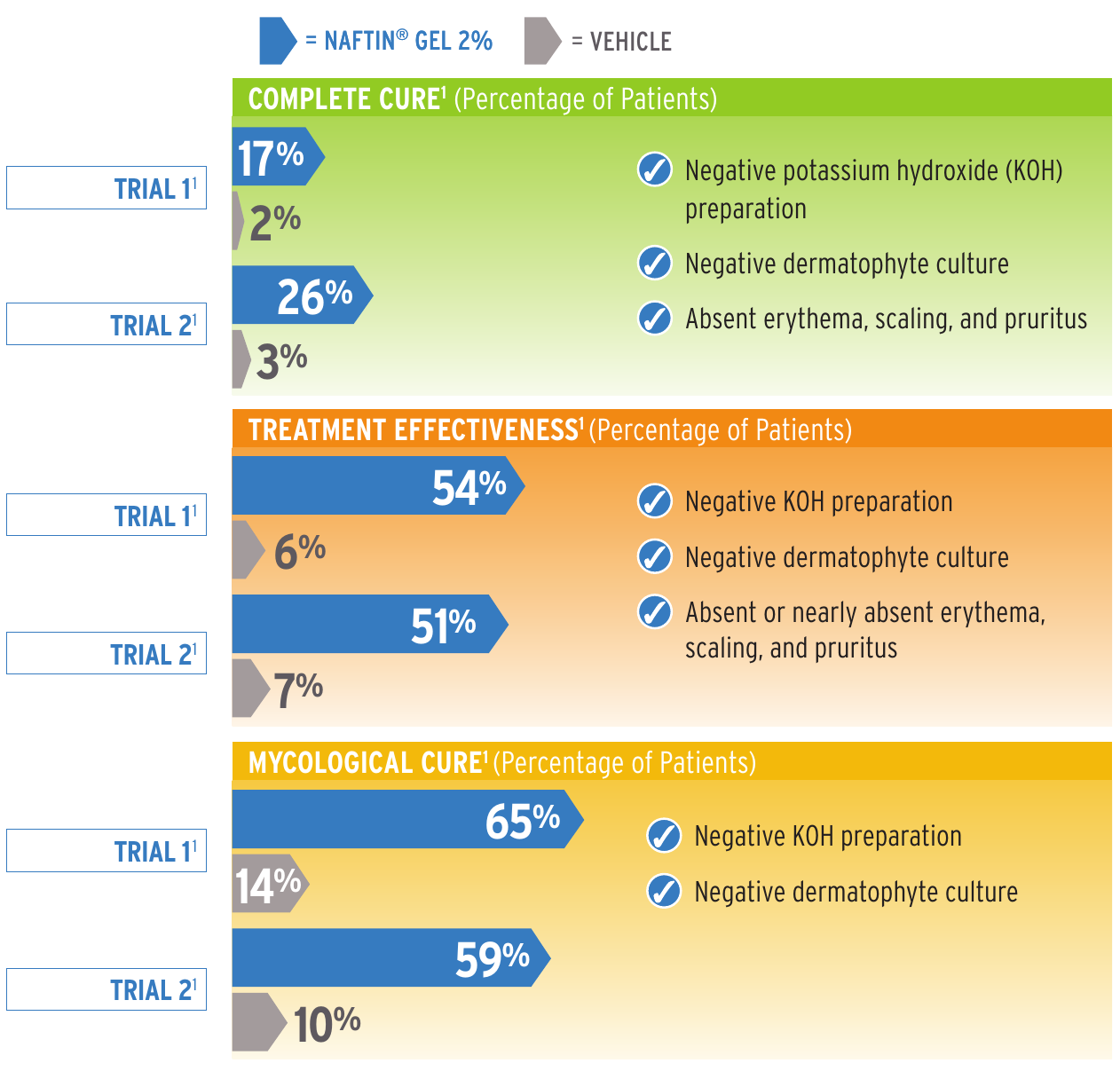
Complete Cure (percentage of patients). Reference: 1.
| Naftin Gel 2% | Vehicle | |
|---|---|---|
| Trial 1 | 17% | 2% |
| Trial 2 | 26% | 3% |
Negative potassium hydroxide (KOH) preparation.
Negative dermatophyte culture.
Absent erythema, scaling, and pruritus.
Treatment Effectiveness (percentage of patients). Reference: 1.
| Naftin Gel 2% | Vehicle | |
|---|---|---|
| Trial 1 | 54% | 6% |
| Trial 2 | 51% | 7% |
Negative KOH preparation.
Negative dermatophyte culture.
Absent or nearly absent erythema, scaling, and pruritus.
Mycological Cure (percentage of patients). Reference: 1.
| Naftin Gel 2% | Vehicle | |
|---|---|---|
| Trial 1 | 65% | 14% |
| Trial 2 | 59% | 10% |
Negative KOH preparation.
Negative dermatophyte culture.